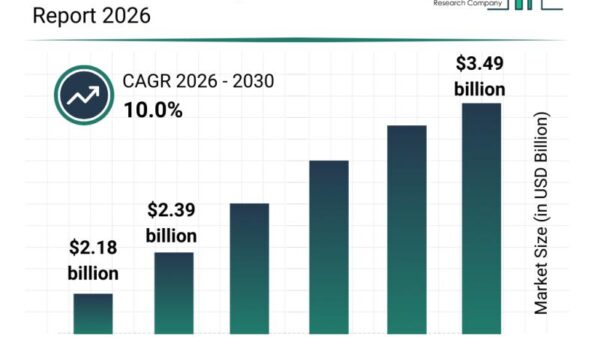
Leveraging AI in Early-Stage Pharmaceutical Development
In the realm of pharmaceutical research and development, the integration of artificial intelligence (AI) and machine learning (ML) is revolutionizing early-stage formulation development. A staggering 90% of drug candidates currently face challenges related to poor solubility, a critical factor that jeopardizes their commercial potential. To counter this issue, advanced computational models are employed in silico to predict optimal solubilization techniques, such as amorphous solid dispersion formulations.
These sophisticated models utilize methods like molecular dynamics simulations and quantum mechanical calculations to analyze molecular descriptors, including potential energy surface charge models and hydrogen bond interactions. By characterizing the interactions between drugs and excipients, these frameworks not only guide the selection of excipients but also minimize the consumption of active pharmaceutical ingredients (APIs) and reduce reliance on traditional trial-and-error methods. This systematic approach ultimately results in significant time and resource savings.
AI-Powered Digital Twins in Preclinical Evaluation
The landscape of preclinical evaluations is evolving as AI-powered digital twins emerge as a transformative solution. Traditional preclinical models are often criticized for their high costs and low translatability to human outcomes. Digital twins—computer simulations that emulate physical systems—are trained using extensive multi-modal data from ex-vivo lung perfusion systems, which provide precise insights into isolated human organs. Remarkably, these models have surpassed 90% accuracy in predicting lung function.
This innovation enables the creation of a personalized digital control arm for each treated organ by generating counterfactual outcomes—essentially predicting what would happen without treatment. Researchers can then conduct paired statistical analyses, facilitating direct comparisons between observed treatment outcomes and those generated by the digital twin. This capability has the potential to uncover therapeutic effects that conventional two-arm studies might overlook, thereby accelerating drug discovery processes while reducing the necessary study size.
Transforming Bioanalytical and Manufacturing Workflows with AI
The role of AI extends beyond formulation development and preclinical evaluations. In bioanalytical workflows and manufacturing, the combination of AI, ML, and large language models (LLMs) is enhancing quality, efficiency, and compliance while simultaneously reducing human error. Current applications include automating report writing and quality control checks, which integrate data from electronic lab notebooks and laboratory information management systems to identify trends in potential failures.
Moreover, LLMs are streamlining the creation of study protocols and validation reports and can even draft sections of electronic common technical documents. AI algorithms also optimize assay method development, including ligand binding and liquid chromatography coupled with mass spectrometry, by predicting optimal conditions and enhancing peak detection capabilities. Regulatory authorities are keenly interested in utilizing AI for analyzing data submitted in filings and during inspections.
In manufacturing, the anticipated impact of AI is profound, with expectations of improved process outcomes such as yield enhancement, increased right-first-time rates, and accelerated technology transfer. AI’s ability to identify patterns across extensive datasets is essential, particularly for complex modalities like cell and gene therapies.
Challenges to Widespread AI Adoption in Pharmaceuticals
Despite these advancements, significant barriers still impede broader AI adoption in the pharmaceutical industry. One of the most pressing issues is the necessity for digitalization, coupled with a lack of high-quality data. Many organizations are often unaware of the extent of fragmentation within their legacy systems until they engage in AI implementation, which necessitates considerable preliminary efforts in data consolidation and cleansing.
Additionally, organizational constraints, including inconsistent AI strategies and the presence of internal silos, further complicate the pathway to AI integration. As regulatory guidance evolves, it emphasizes a risk assessment approach that evaluates how AI models influence the quality, safety, and efficiency of final drug products. For regulated bioanalytical processes, controls must be established to mitigate risks such as “hallucinations,” or the generation of non-existent data, which necessitates robust audit trails to ensure compliance.
In conclusion, while the integration of AI in pharmaceutical development presents exciting opportunities, addressing these challenges is crucial for unlocking its full potential. The pharmaceutical sector stands at a pivotal moment where AI can significantly enhance drug discovery and development, provided that the hurdles of data quality and organizational readiness are effectively managed.
 AI Experts Discuss Economic Impact of AI on Industries in Live Roundtable Event
AI Experts Discuss Economic Impact of AI on Industries in Live Roundtable Event East Bay Man Charged with Conspiracy to Smuggle $4M in Nvidia AI Chips to China
East Bay Man Charged with Conspiracy to Smuggle $4M in Nvidia AI Chips to China Case Western Reserve Secures $2.5M Grant to Enhance AI Technology for Tracking Cancer Cells
Case Western Reserve Secures $2.5M Grant to Enhance AI Technology for Tracking Cancer Cells NVIDIA Achieves #1 Spot on Forbes America’s Dream Employers 2026 List Amid AI Surge
NVIDIA Achieves #1 Spot on Forbes America’s Dream Employers 2026 List Amid AI Surge Min Chun Fu Joins Comp AI as Co-Founder to Enhance User-Centric AI Compliance Systems
Min Chun Fu Joins Comp AI as Co-Founder to Enhance User-Centric AI Compliance Systems